After years of anticipation, sodium-ion batteries are starting off to produce on their assure for energy storage. But so far, their commercialization is confined to massive-scale employs these types of as storing energy on the grid. Sodium-ion batteries just never have the oomph essential for EVs and laptops. At about 285 Wh/kg, lithium-ion batteries have two times the energy density of sodium, generating them much more appropriate for these portable apps.
Researchers now report a new type of graphene electrode that could enhance the storage capability of sodium batteries to rival lithium’s. The product can pack almost as many sodium ions by quantity as a common graphite electrode does lithium. It opens up a route to generating small-price tag, compact sodium batteries realistic.
Abundant and low cost, and with similar chemical houses as lithium, sodium is a promising substitution for lithium in up coming-era batteries. The security and protection of sodium batteries will make them primarily promising for electronics and vehicles, in which overheated lithium-ion batteries have at times established harmful.
“But at present the important difficulty with sodium-ion batteries is that we never have a appropriate anode product,” states Jinhua Sunlight, a researcher in the section of industrial and supplies science at Chalmers College of Know-how.
For the battery to demand immediately and retail outlet a whole lot of energy, ions will need to easily slip in and out of the anode product. Sodium-ion batteries use cathodes made of sodium metallic oxides, while their anodes are generally carbon-dependent anodes just like their lithium cousins while Santa Clara, California-dependent Natron Electrical power is generating the two its anodes and cathodes out of Prussian Blue pigment employed in dyes and paints.
Some sodium battery builders are employing activated carbon for the anode, which holds sodium ions in its pores. “But you will need to use significant-quality activated carbon, which is really high-priced and not effortless to produce,” Sunlight states.
Graphite, which is the anode product in lithium-ion batteries, is a lower price tag possibility. Having said that, sodium ions do not move competently involving the stack of graphene sheets that make up graphite. Researchers employed to think this was mainly because sodium ions are even bigger than lithium ions, but turns out even-even bigger potassium ions can move in and out easily in graphite, Sunlight states. “Now we think it can be the surface area chemistry of graphene layers and the digital structure that simply cannot accommodate sodium ions.”
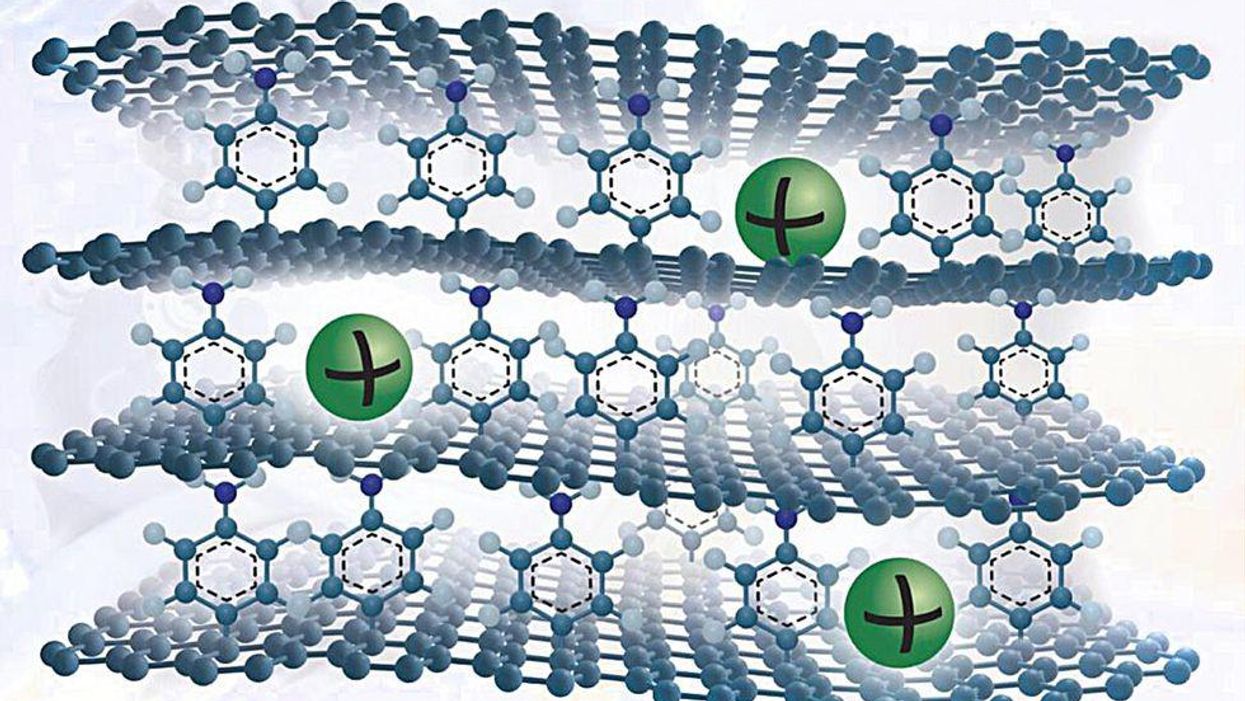
He and his colleagues have occur up with a new graphite-like product that overcomes these concerns. To make it, they grow a solitary sheet of graphene on copper foil and attach a solitary layer of benzene molecules to its top surface area. They grow many these types of graphene sheets and stack them to make a layer cake of graphene held apart by benzene molecules.
The benzene layer increases the spacing involving the layers to permit sodium ions to enter and exit easily. They also produce flaws on the graphene surface area that as as lively reaction web sites to adsorb the ions. Moreover, benzene has chemical teams that bind strongly with sodium ions.
This seemingly simple system boosts the material’s sodium ion-storing capability substantially. The researchers’ calculations display that the capability matches that of graphite’s capability for lithium. Graphite’s capability for sodium ions is generally about 35 milliAmpere-several hours per gram, but the new product can maintain around 330 mAh/g, about the very same as graphite’s lithium-storing capability.
